
Secondly, this section specifies the role of top management in the QMS.

This is performed according to specific instructions documented in quality procedures.įor instance, Section 4 describes as well the requirements for the Medical Device File (the equivalent of the Design Master Record for US QSR). A QMS is a series of processes which transform inputs in outputs. Section 4: Quality Management Systemįirstly, this section specifies the requirements for implementation of QMS processes and document management system. ISO Guide 73 - 2009 - Risk management - Vocabulary.Currently, there are 5 sections that contain all the requirements of ISO 13485, specifically between section 4 and section 8 of the standard.ISO 14971:2007 Medical devices Application of risk management to medical devices.Risk Management is a requirement: Product Realization clause 7.1 o See guidance standards.Buy copies of the ISO13485 standard & the FDA QSR (21 CFR 820) regulation to pinpoint the areas that need attention.Comparison between FDA QSR -and-ISO-13485:.Links to buy Standards directly from the source (TechStreet) are Underlined Bold Red text Here are some resources you will want to complete your Gap Analysis:.Links to supporting information are underlined blue text.
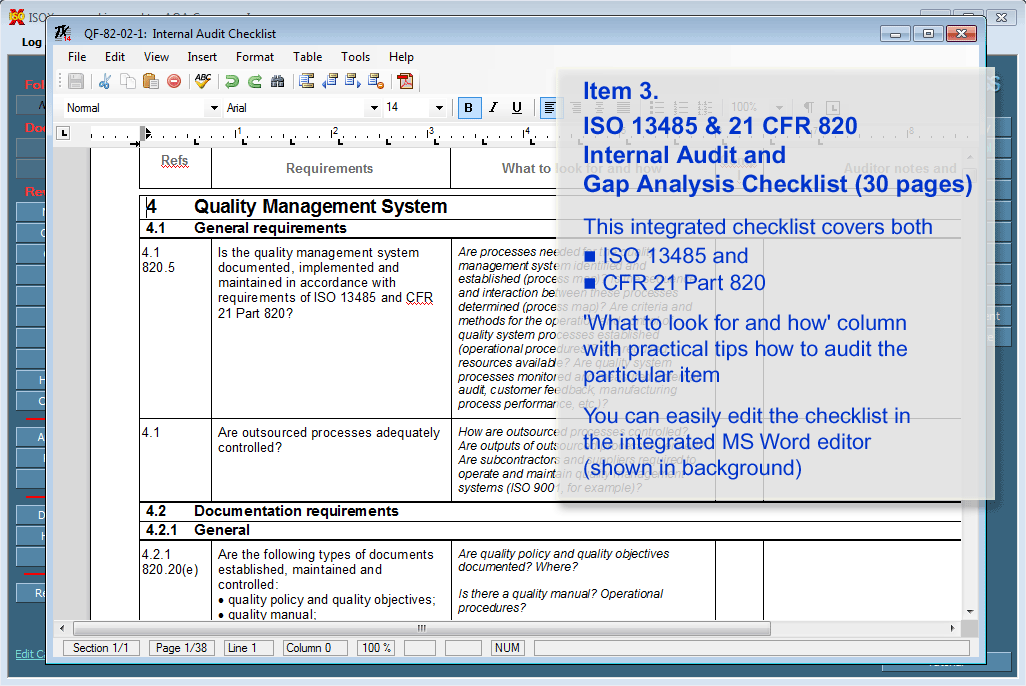
ISO 13485:2003/FDA QSR - INTERNAL AUDIT CHECKLIST The checklist provides questions that refer to the ISO 13485: 2003 standard.


 0 kommentar(er)
0 kommentar(er)
